IMI (International Medical Industries, Inc.) brings expertise in Secure Drug Delivery to Arab Health 2021
{June 14, 2021} IMI (International Medical Industries, Inc.) brings expertise in secure drug delivery to Arab Health 2021 with the expansion of their line of tamper evident products.
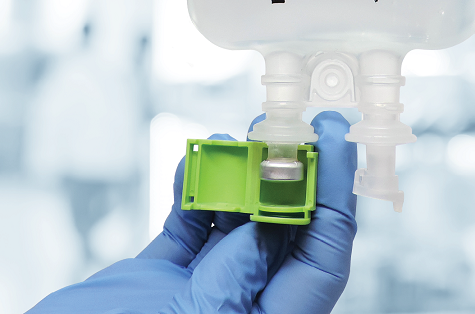
IMI is the industry leader in tamper-evident products that enhance medication safety throughout the pharmaceutical supply chain. IMI’s Prep-Lock™ Brand of Tamper Evident products includes security devices for IV, Oral, and Enteral syringes, IV bags, and CADD Medication Cassettes.
Over 86% of the top outsource compounders in the US choose Prep-Lock Tamper Evident Caps from IMI to secure their IV Syringes. Recently IMI introduced two new products into this popular line; the Tamper Evident Additive Port Cap for B.Braun IV Bags and Tamper Evident Additive Port Cap for Fresenius Kabi IV Bags. These products will be added to the existing line of Additive Port Caps that provide protection for Baxter Viaflex, Intravia, Avivia, and All-In-One EVA bags.
In addition to an active deterrent to drug diversion and misuse, tamper evident products help ensure medication safety and sterility from pharmacy to patient.
Numerous organizations have published guidelines recommending the use of tamper evident products to safeguard the integrity and security of medications including the American Society of Health-System Pharmacists (ASHP), the United States Food & Drug Association (FDA) and the United States Pharmacopeia (USP). “All Packaging should be somewhat tamper-evident,” says Dr. Paul Stranges, PharmD, BCACP, AE-C Clinical Assistant Professor at the Department of Pharmacy Practice at The University of Chicago’s College of Pharmacy. “Outside of diversion it provides reassurance the product contains what is labeled and is sterile. Use of tamper-evident packaging gives pharmacies, healthcare work personnel and patients more confidence.” All tamper-evident products by IMI are designed with the pharmacist in mind, delivering exceptional ease of use, peace of mind and strong cost/benefit risk mitigation. The Additive port cap provides a solid, secure closure to the additive port of IV Bag medications with simple one-handed installation.
Most importantly, according to Neil Colby, RPh Director of Infusion Pharmacy Services as CDRx Infusion, “You feel confident that your IV bags are not going to be tampered with.” He continued, “I find their product to be the best on the market in terms of the device itself, the functionality and the securement.” IMI representatives will be live in the USA Partner Pavilion H1.E14, June 21st through June 24th 10:00 – 18:00 discussing the benefits of incorporating IMI tamper evident technologies and providing live demonstrations on their ease of use. IMI is currently seeking international distribution partnerships, please contact IMI directly for available opportunities.
Book your appointment today through Arab Health Online or visit Booth H1.E14 for more information.
In addition to industry-leading tamper evident products IMI also offers a comprehensive portfolio of essential pharmacy compounding devices such as connectors, caps, plugs, aspirating needles and venting needles.
For more information about IMI visit https://IMIweb.com