RGF® Environmental Group HALO-LED™ Proven to Reduce Coronavirus Surrogate MS2 Bacteriophage by 99% in Independent Testing
Successful certified testing conducted using an air-sampling model proves virus destroying power
Port of Palm Beach, FL | 17 September 2021
RGF® Environmental Group, Inc., a leading environmental design and manufacturing company, has released the results of a third-party study that has proven the effectiveness of HALO-LED™ technology in the reduction of MS2 Bacteriophage, a surrogate for SARS-CoV-2 (COVID-19), indoor environments.
There is mounting research to suggest that any microorganism, including viruses, can become airborne. Contaminated material can be aerosolized in many different ways, ranging from wind to human and animal activities such as sneezing, coughing, talking, mechanical processes, etc. If the aerodynamic size of an infectious particle is appropriate, it can remain airborne, come into contact with humans or animals, and potentially cause an infection. Airborne microorganisms can represent major health and economic risks to human and animal populations.
The HALO-LED™ by RGF® is the industry’s first LED in-duct, whole home and building air purification system that is both mercury free and verified zero ozone compliant. The HALO-LED™ proactively treats every cubic inch of air-conditioned space, reducing airborne and surface contaminants and pollutants through bi-polar ionization and revolutionary, patent pending, REME-LED™ technology.
Overseen by Dr. James Marsden, Executive Director of Science and Technology at RGF®. “The effective reduction of airborne virus is a major breakthrough in the battle to control SARS-CoV-2 in indoor environments. As it shows the HALO-LED™ to be effective in combating the MS2 Bacteriophage virus and a valuable solution to immediately improve the Indoor Air Quality of residential and commercial spaces and protect occupants against exposure to the surrogate SARS-CoV-2 virus from this second wave.” The studies are ongoing.
The study was conducted at Intertek, an accredited independent microbiology laboratory in Columbus, OH. A HALO-LED™ in-duct air purification device was provided by RGF® Environmental for use in the microbial reduction rate test. The microorganism used in the study was MS2 bacteriophage, a small non-enveloped RNA virus (ATCC number 15597-81). It is the preferred surrogate for SARS-CoV-2, the virus that causes COVID-19.
The test chamber measured 10’x10’x10’ or 1,000 cubic feet. The MS2 microbial suspension was aspirated into the chamber. The temperature of the test chamber was maintained at 21° C and relative humidity at 41%.
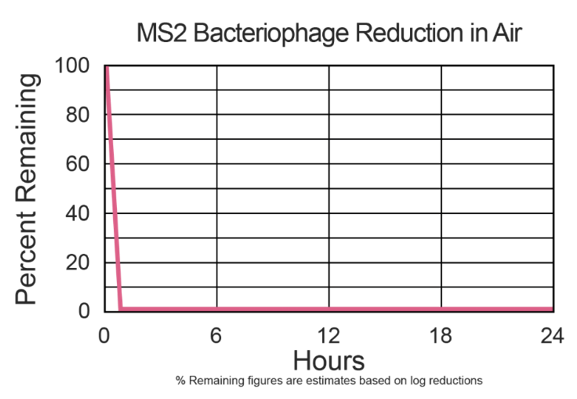
Air samples were taken from the test chamber once the unit was turned on and sampling was taken every 15 minutes over a period of 2 hours, and then plated. The process was then repeated without the test unit in the chamber to provide the natural decay results. All plates were incubated overnight and viral growth on the test plate was compared to that of the natural decay control.
Air sampling took place using an SKC BioStage single-stage impactor for 30 seconds at 12L/min (0.424cubic feet/min). Results shown represent the percent reduction at 120 minutes.
The percent reduction associated with the HALO-LED™ treatment was 99.9% after two hours of exposure.
Testing summary: 99.9% inactivation of the airborne MS2 Bacteriophage within 1,000 cubic feet chamber using an air-sampling model.
DISCLAIMER: The summary and any comments herein are based on the results from an independent laboratory study performed under controlled conditions and are not in any way medical claims. The product(s) and technologies described are not medical devices and are not intended to diagnose, treat, cure, or prevent any disease, virus or illness.