iBreastExam - US born innovation scaling globally, arrives in the Middle East
Product Showcase & Launch at Booth# H1.C31 in the US Pavilion
Breast cancer is the most common cancer in women worldwide and the same is true for women in the United Arab Emirates. Breast cancer is highly treatable when detected early. However, by the time symptoms present, it’s often too late; most women are diagnosed late.
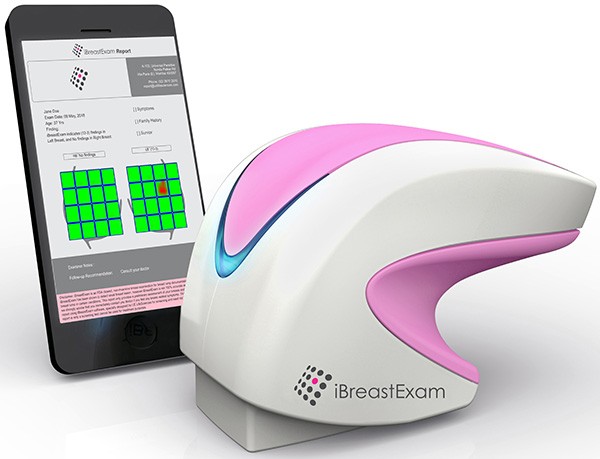
iBreastExam (iBE) is an innovative, handheld medical device designed to provide a quick, painless and radiation-free breast exam at the point-of-care, with instant results. It is meant to be used by community health workers, nurses and allied health professionals in primary care centers, gynecology clinics and women’s health providers as a pre-screening tool.
With a commitment to bring down cancer fatalities by nearly 18 per cent by 2021, the UAE is taking the challenge of cancer detection head-on. In fact, reducing the number of deaths due to cancer is one of the key performance indicators of the pillar of world-class healthcare of the UAE National Agenda.
Bhaumik Sanghvi, UE LifeSciences’ Chief Operating Officer, said “We are very excited to bring this technology to the women in the Middle East! It’s for healthy women, before symptoms of breast cancer appear. Breast tumors are hard, like a seed inside the lemon. Our patented ceramic sensors in iBE measure tissue elasticity in real-time by converting mechanical pressure into electrical signals. For women, it feels like a stethoscope on the breasts. It’s a quick and easy test.”
Over 200,000 women have taken the iBreastExam test in over 12 countries. The device has received commercial market clearance by regulatory authorities in the United States (US FDA), Europe (CE Mark), Mexico (COFEPRIS), India and several Southeast Asian countries. In various clinical studies, over 10,000 women have been enrolled to study iBreastExam extensively. iBE has demonstrated sensitivity of 84%, specificity of 94% and negative predictive value of 98%.
iBreastExam recently launched commercially in Oman with the support of Oman Health Ministry and Oman Cancer Society, and the next stop is the UAE. UE LifeSciences is in talks with major providers and seeking collaborations with clinics, diagnostics and hospital players, and the government.
“We are an innovation led US company with a global DNA” said Prof. Matthew Campisi, UE LifeSciences’ Co-founder and Chief Technology Officer. “With devices that are on the ground and projects that are ongoing already, over 1 million women will soon receive a much needed, safe, effective and affordable breast exam. For most women it will be for the very first time”, said Prof. Campisi.
iBreastExam has been featured in TED Talks, New York Times, BBC News, Vogue Magazine and others. The innovation has also won several international innovation awards and over $1.6M in grant funding from the Bayer Cares Foundation, Pfizer Foundation and the Pennsylvania Department of Health.
** About UE LifeSciences **
With "Impact Innovation” as the guiding mission, UE LifeSciences (UELS) is a fast growing, medical device company commercializing innovative science into clinically effective and affordable mHealth cancer detection tools for parts of the world where the burden of cancer is dramatically rising and where access to early detection remains significantly limited.
UELS has commercialized 2 US FDA cleared and CE marked products for early detection of breast cancer, and has raised $4.2M in equity capital and $1.6M in grant capital, including the prestigious CURE Grant award from Pennsylvania Dept. of Health. UELS has offices in USA, India and Malaysia, with a team of over 75 people.
For further Media/Business/Clinical Inquiries, please contact us at:
[email protected]
+1.631.980.8340 Americas
+60.192109245 Asia
+91.22.2610.2610 Middle East